The INNODERM Project
The Mission
INNODERM is an EU funded project that brings together the expertise of Small and Medium Size Enterprises (SMEs) and academic partners the Technical University of Munich (TUM), Humanitas University Milan (HUNIMED), iThera Medical GmbH, Rayfos Ltd, and Sonaxis SA in the fields of photonic and ultrasound technologies.
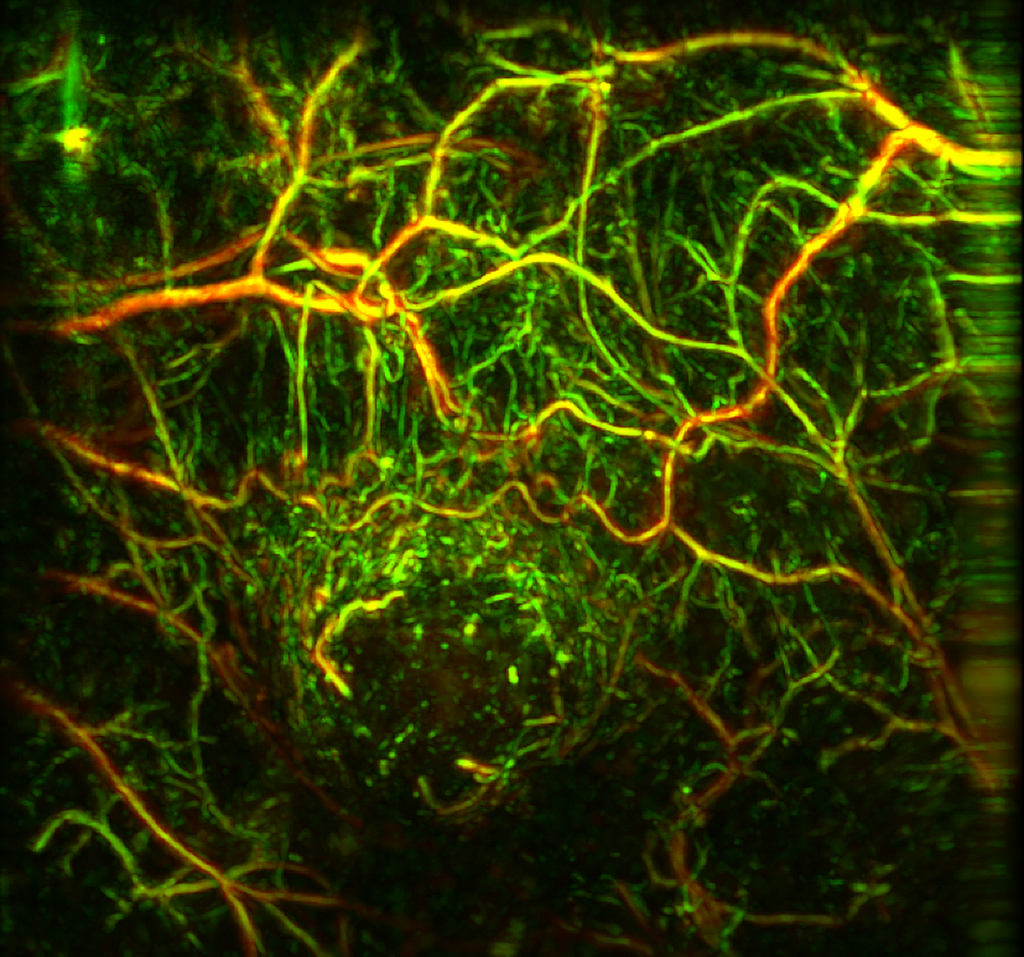
RSOM image of tumor vasculature in a mouse model.
The goal of INNODERM has been to develop a novel Raster-Scan Optoacoustic Mesoscopy (RSOM) device to improve non-invasive diagnostics for skin cancer and other dermaological conditions.
RSOM is based on Multispectral Optoacoustic Tomography (MSOT), a proven imaging method that generates high-resolution optical images in scattering media and dramatically improves upon conventional bio-optic barriers by enabling (1) three-dimensional high-resolution optical imaging deep inside tissues (up to several centimeters), (2) high-scalability, ranging from optical-resolution microscopy to acoustic-resolution optical mesoscopy and macroscopy, and (3) novel label-free anatomical, physiological and molecular contrast at the tissue and single-cell-level, based on spectrally-resolved optical absorption. MSOT technology is commercialized by iThera Medical for macroscopy imaging, while RSOM was developed to image features at the meso- and micro- scopic levels.
INNODERM aimed and succeeded to design, manufacture, and validate the technical viability of a handheld, portable, scalable label-free RSOM prototype for point-of-care applications in dermatology diagnostics and skin disease monitoring. RSOM goes beyond the abilities of other current optical or optoacoustic devices and offers a paradigm shift in dermatology imaging.
Objectives
Demonstration of clinical RSOM imaging system. Video courtesy of iThera Medical GmbH.